42 fda requirements food labels
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Food Labeling Requirements - TheMarketatdelval.com The USDA's food labeling requirements are designed to provide information about the food product, including the name of the product, the name and address of the manufacturer or distributor, the net quantity of contents, and the nutrition facts panel.
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
Fda requirements food labels
What are the FDA requirements for food- USA Food regulations Product labeling is one of the most important FDA requirements for food products. Labeling is one of the important FDA requirements for food products. We can offer labeling review services and our fee for per product labeling review is $ 299. In order to review the label, you should provide us label design in PDF or image format. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the common or usual name of each ingredient ... FDA Food Label Requirements - Graphics Universal Incorporated "Folate" and "Folic Acid" must be used for purposes of declaration in the labeling of conventional foods and dietary supplements. The declaration for folate must be in mcg DFE (when expressed as a quantitative amount by weight in a conventional food or a dietary supplement), and percent DV based on folate in mcg DFE.
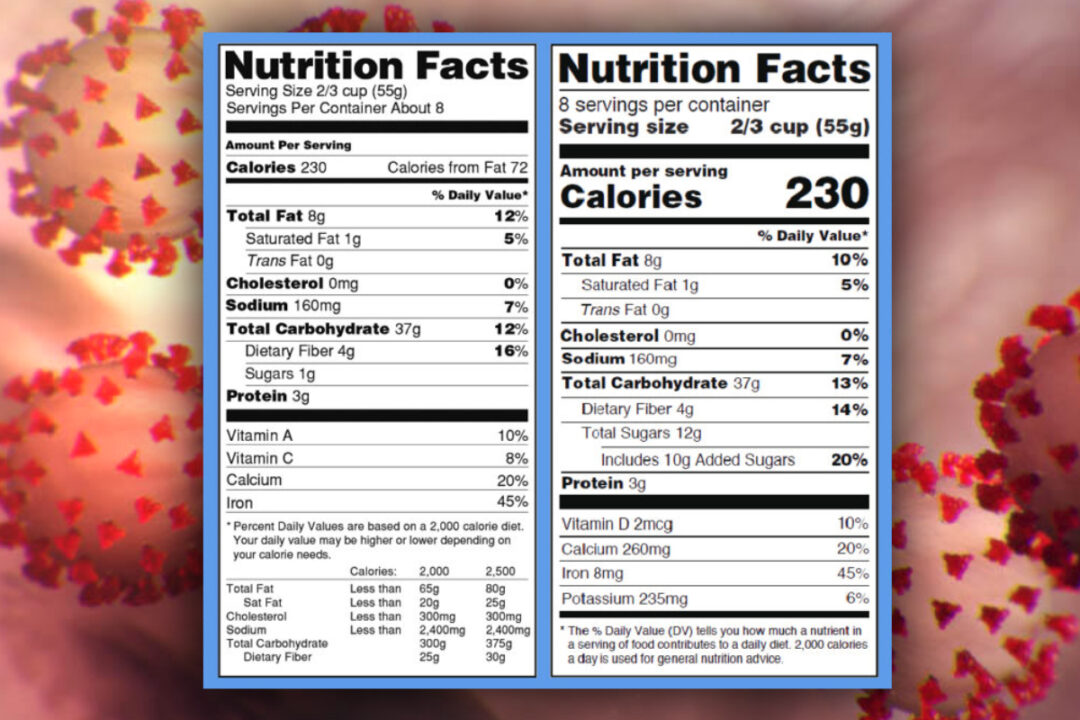
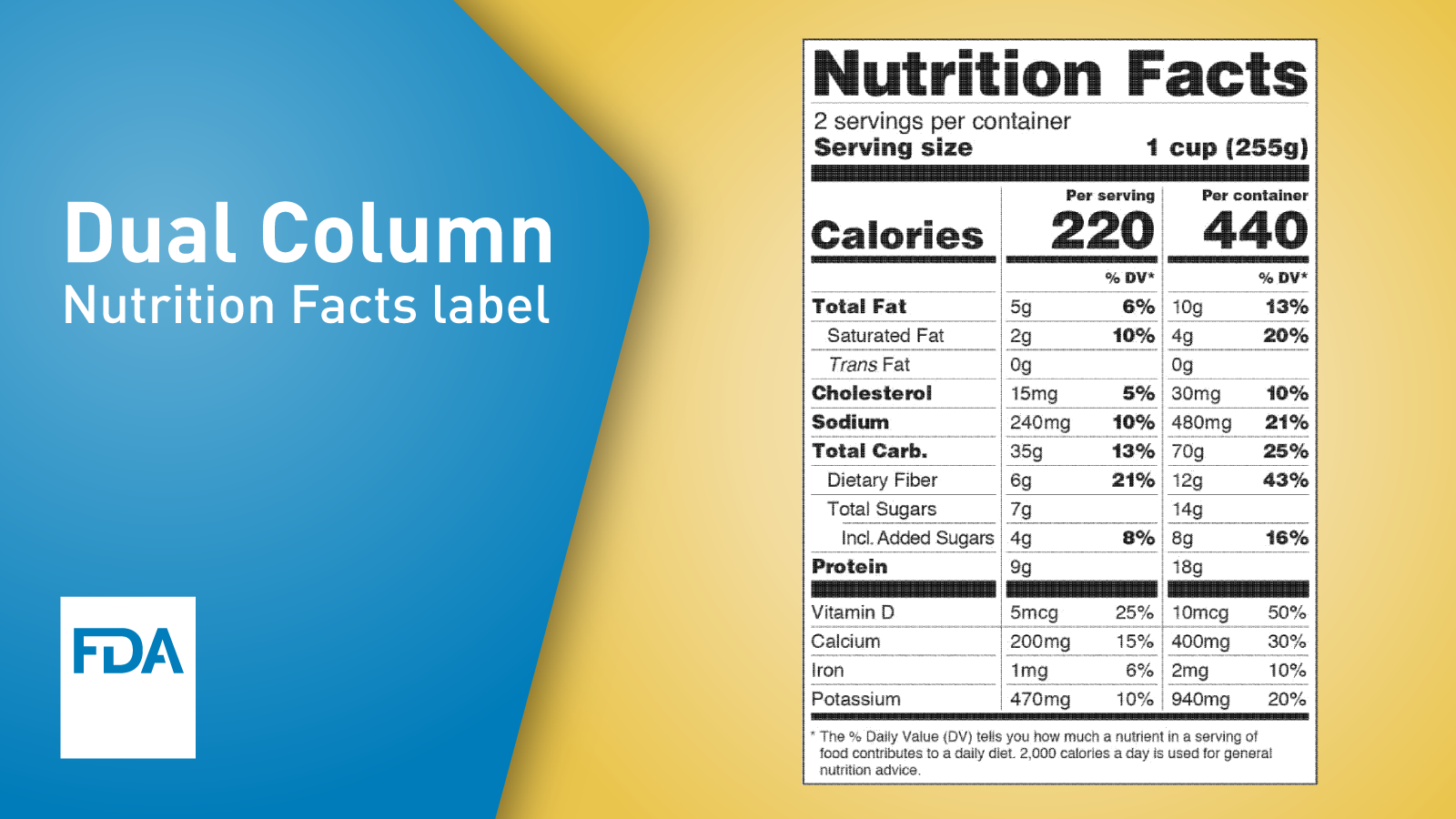
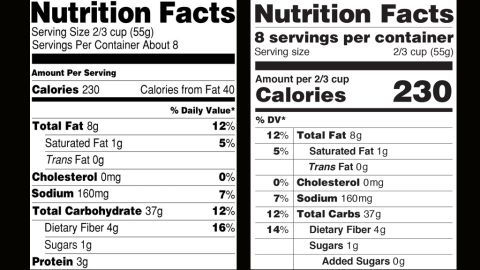
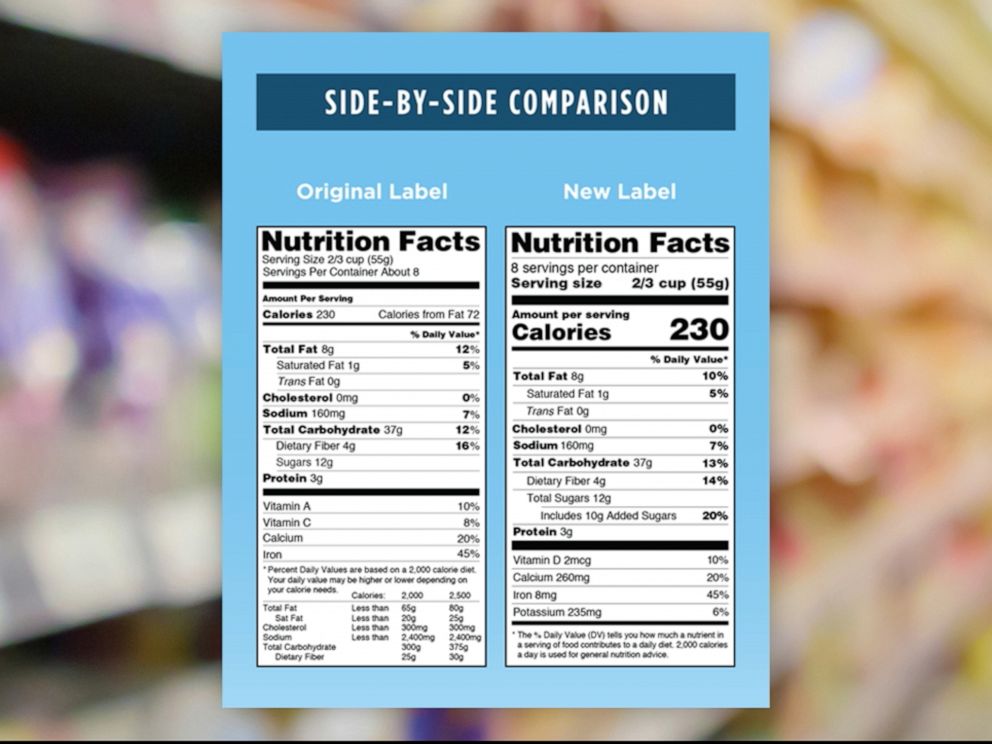
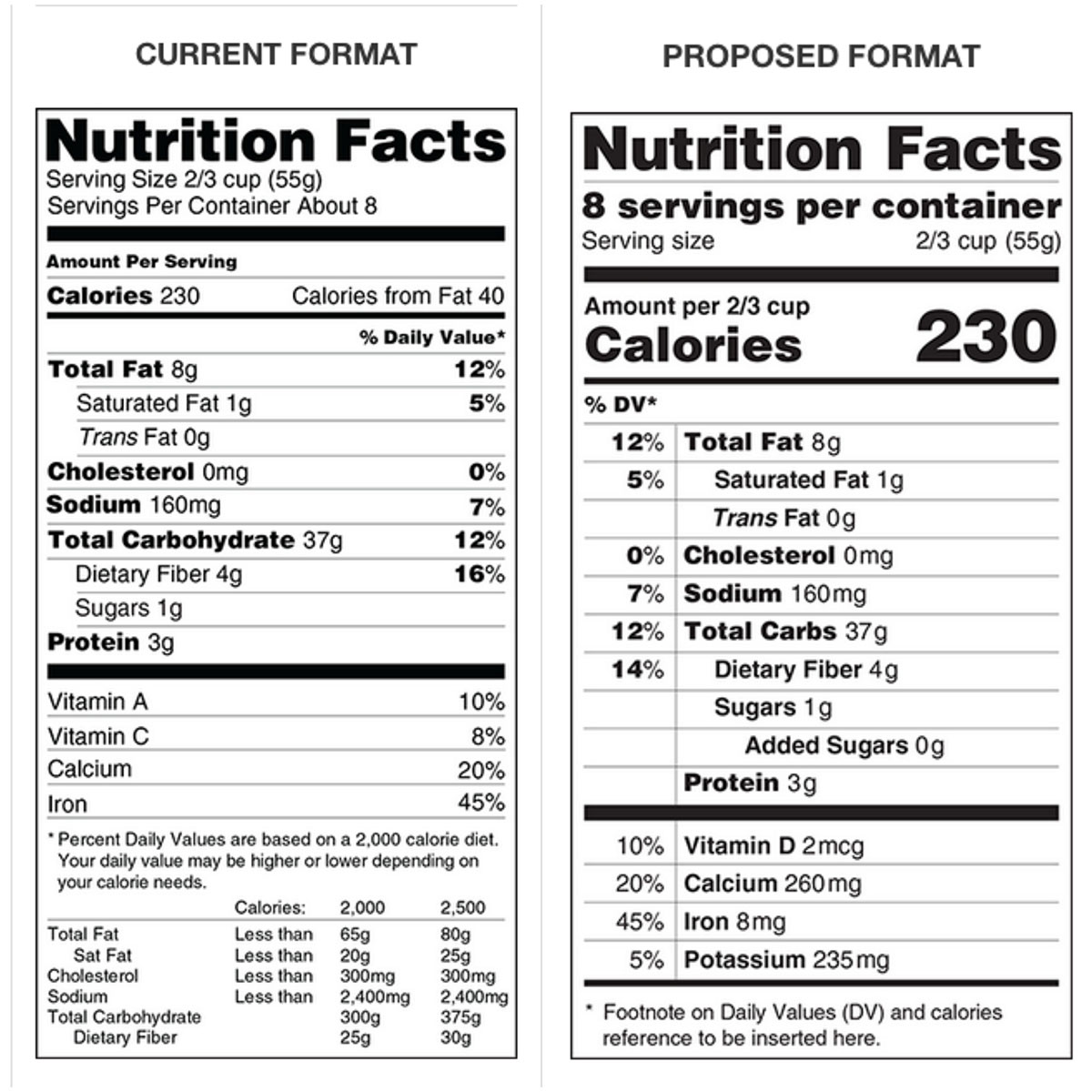
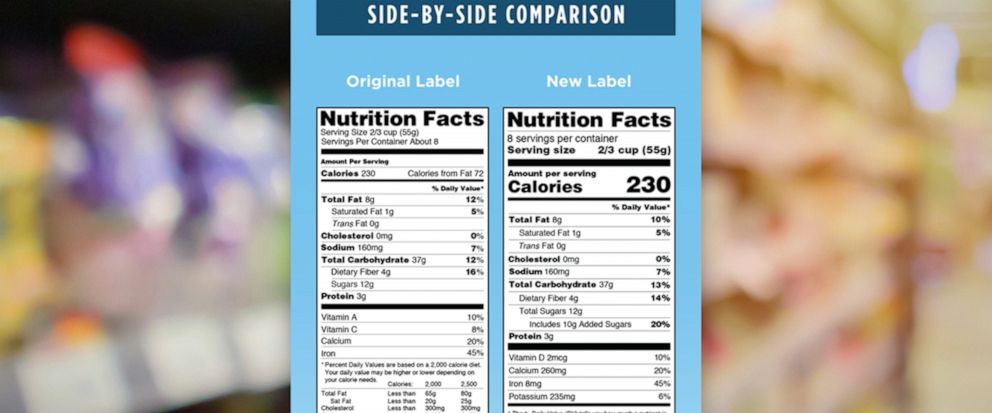
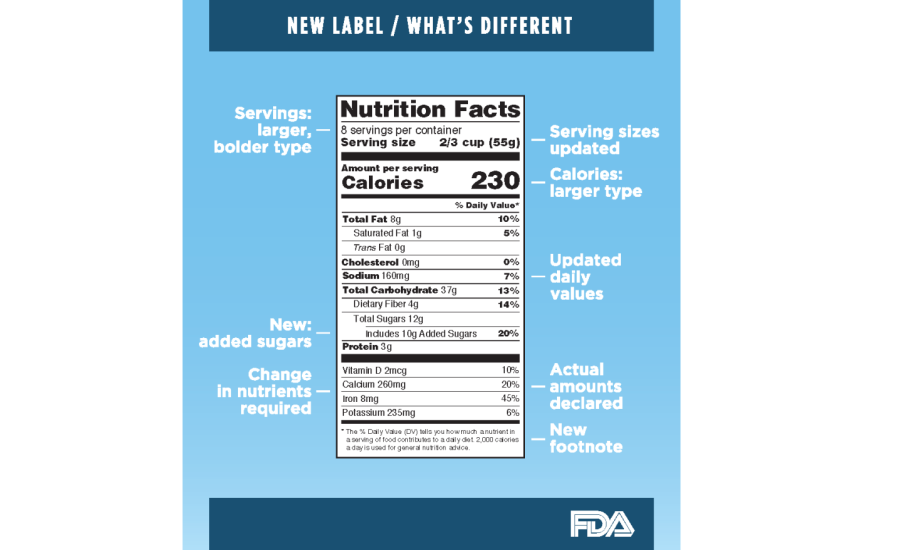
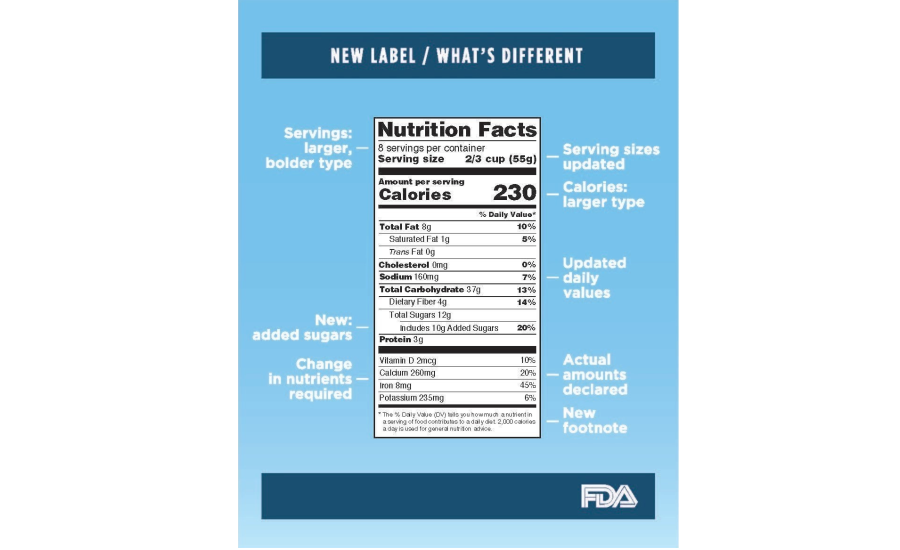
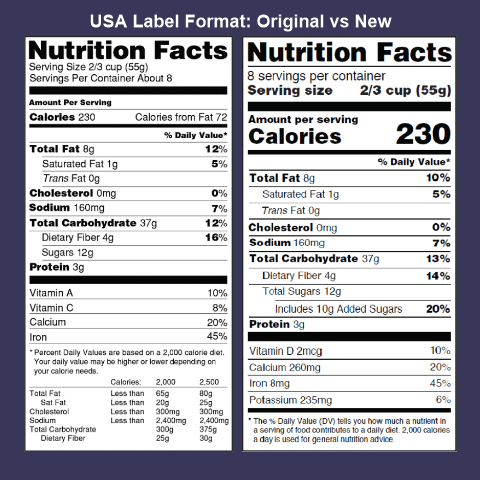
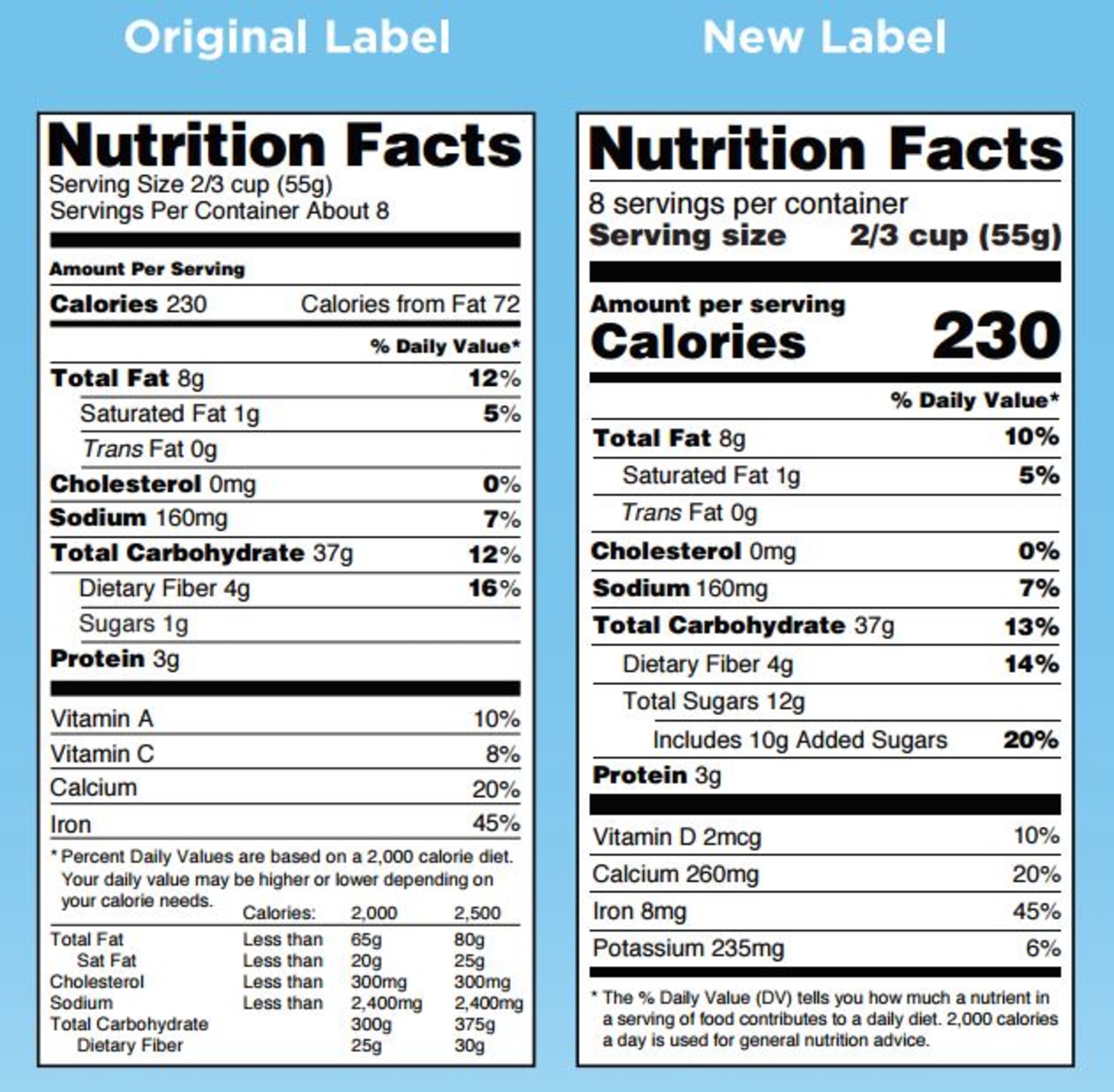
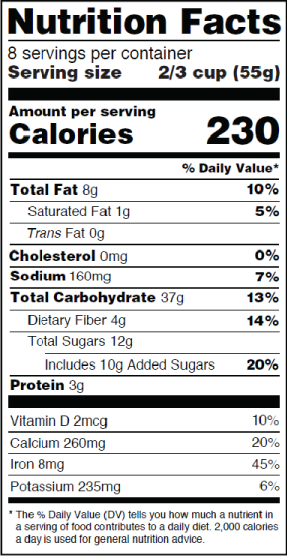
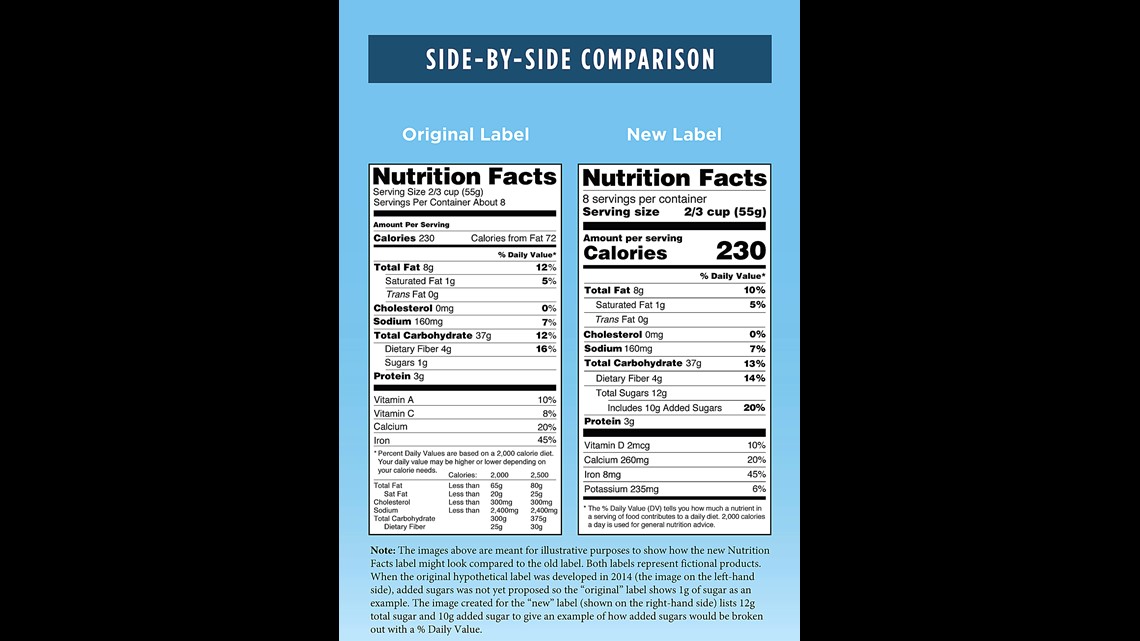
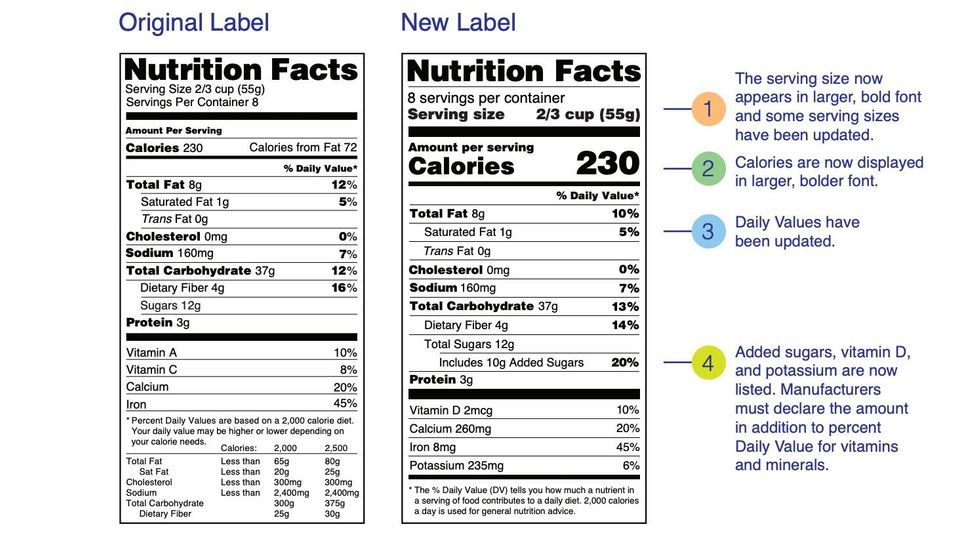
Fda requirements food labels. The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.7 Declaration of net quantity of contents. (a) The principal display panel of a food in package form shall bear a declaration of the net quantity of contents. This shall be expressed in the terms of weight, measure, numerical count ... Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (e) If a person manufactures, packs, or distributes a food at a place other than his principal place of business, the label may state the principal place of business in lieu of the actual place...
PDF Food Labeling Guide - Food and Drug Administration Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740 (Tel)... Guidance for Industry: Food Labeling Guide | FDA It is the responsibility for the food industry to remain current with the legal requirements for food labeling. All new regulations are published in the Federal Register (FR) prior to their... Fda packaging and labeling requirements - vhvi.fuhrerscheinekaufen.de The Philippines' Food and Drug Administration (FDA) issued Administrative Order No. 2014-0030, or otherwise known as the Revised Rules and Regulations. Labels must have basic information regarding the manufacturer's name and address, expiration date, a listing of ingredients, lot number, and the product form, e.g., powder, liquid, etc. Menu Labeling Requirements | FDA - U.S. Food and Drug Administration The menu labeling requirements apply to restaurants and similar retail food establishments that are part of a chain with 20 or more locations. In addition, they must be doing business under the...
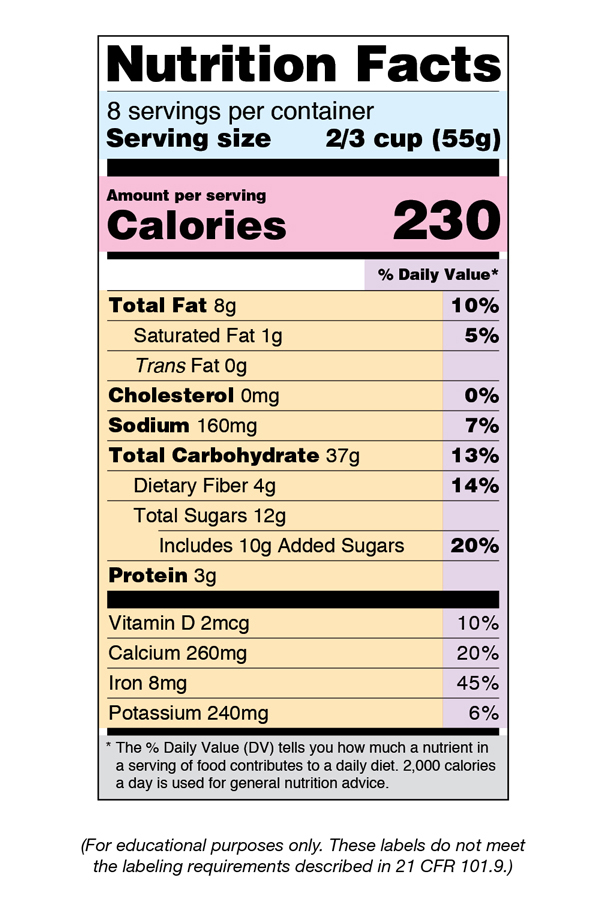
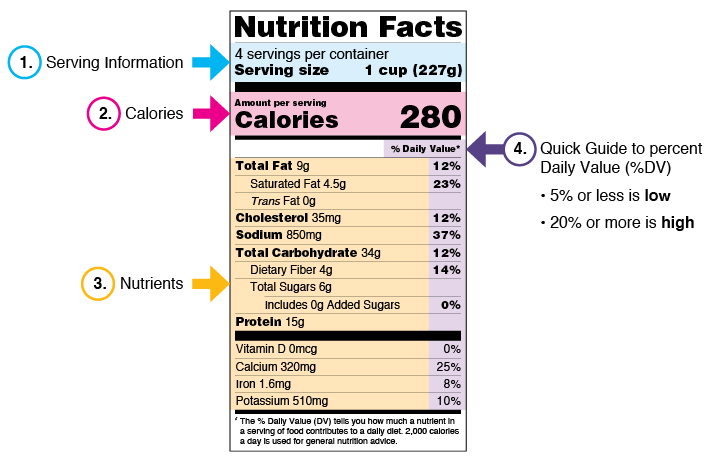
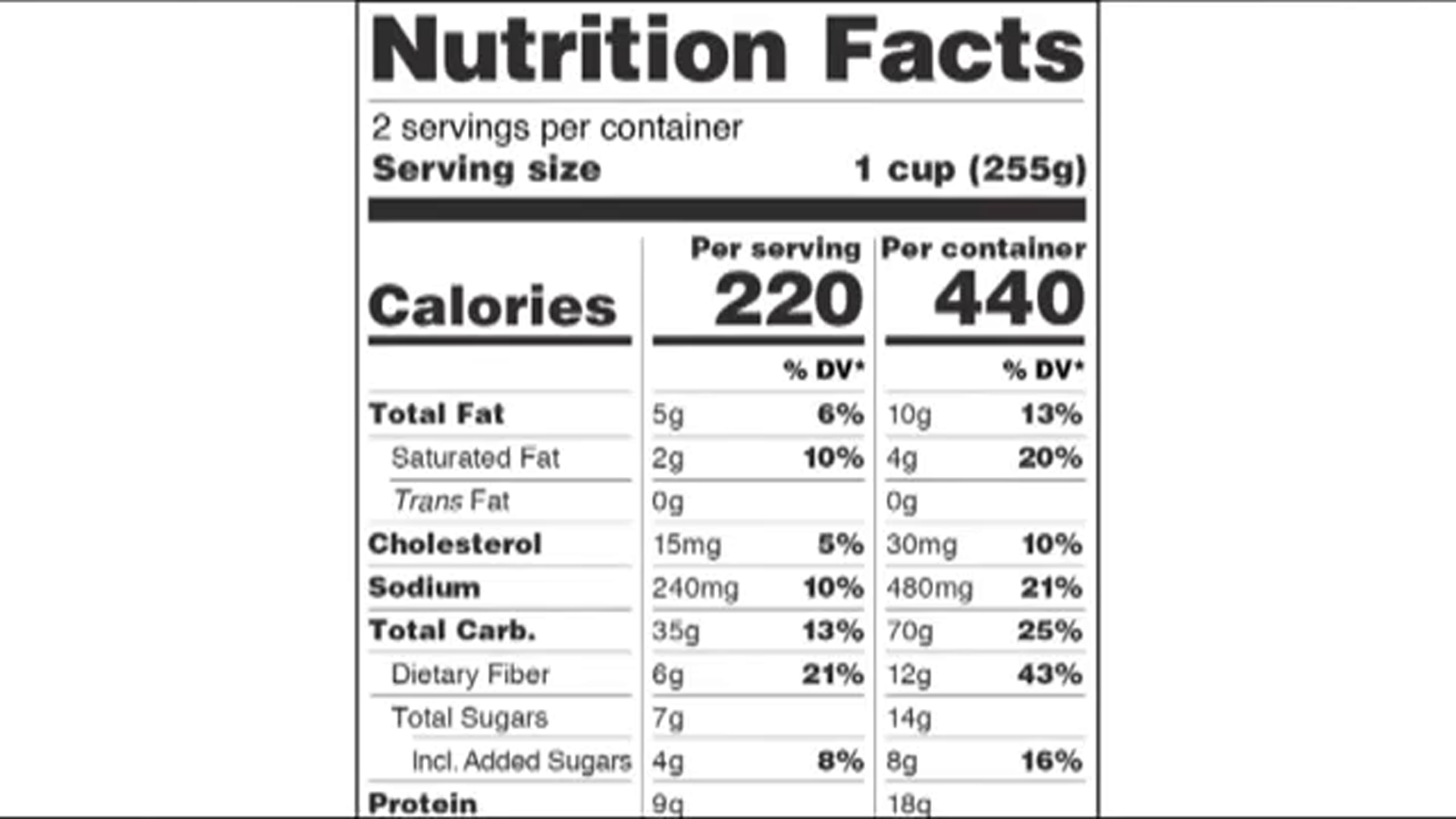
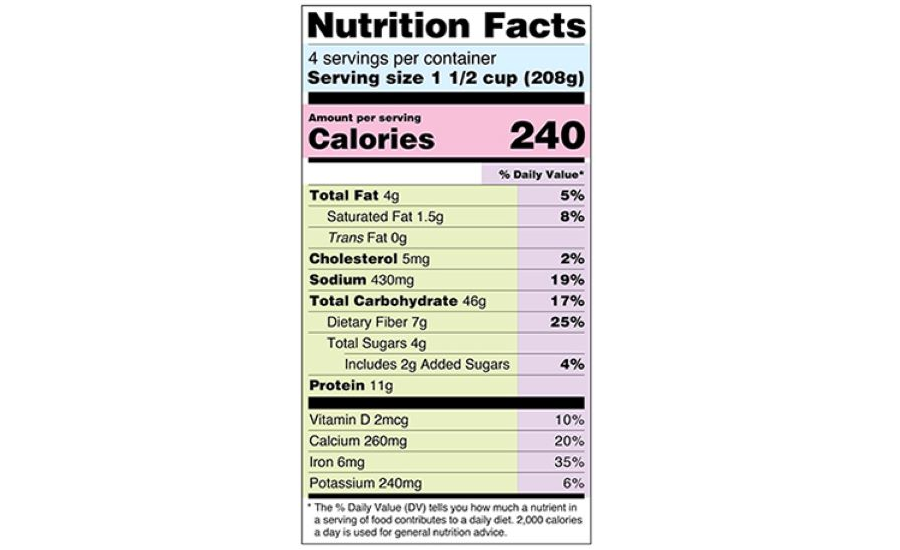
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ... FDA Food Product Labeling & Packaging Requirements - ESHA Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) Placement: In general, place the Nutrition Facts Label on the PDP or the Information Panel, near the ingredient statement. Ingredient Statement Food Labeling - USDA The FDA assures that most prepared foods, such as bread, cereals, canned and frozen foods, snacks, desserts, drinks, etc., are safe, wholesome, and properly labeled. The National Organic Program (NOP), a regulatory program housed within the USDA Agricultural Marketing Service, develops organic food labeling standards CFR - Code of Federal Regulations Title 21 - Food and Drug Administration All approved material is available for inspection at the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-2404 and is available from the sources indicated below.
Food Ingredients & Packaging | FDA FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in meat, poultry, vegetables and...
Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings
Wholesale/Manufactured Food Program Labeling Requirements The FDA has created its Guidance for Industry: Food Labeling Guide to assist in answering the many questions from manufacturers, distributors and importers about the proper labeling of their food products. This guidance is a summary of the required statements that must appear on food labels under current laws and their regulations.
Food Labeling Requirements for FDA Compliant Label Design - enKo Products The FDA requires the following business details on food labels: Business name Street address City or town State Zip Code Indicate if the business name is that of the manufacturer, distributor, importer, etc. If the product is exported or manufactured outside of the US, the country of origin must appear conspicuously on the label for food safety.
FDA Food Label Requirements - Graphics Universal Incorporated "Folate" and "Folic Acid" must be used for purposes of declaration in the labeling of conventional foods and dietary supplements. The declaration for folate must be in mcg DFE (when expressed as a quantitative amount by weight in a conventional food or a dietary supplement), and percent DV based on folate in mcg DFE.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the common or usual name of each ingredient ...
What are the FDA requirements for food- USA Food regulations Product labeling is one of the most important FDA requirements for food products. Labeling is one of the important FDA requirements for food products. We can offer labeling review services and our fee for per product labeling review is $ 299. In order to review the label, you should provide us label design in PDF or image format.




















Post a Comment for "42 fda requirements food labels"